The FDA recently announced a landmark change to biosimilar development: clinical efficacy studies are no longer required in many cases. Instead, developers can rely on strong bioanalytical, pharmacokinetic (PK), and immunogenicity data to establish biosimilarity.
This shift is a major win for companies that excel in deep product characterization. But it also puts increased pressure on pharma and biotech teams to ensure that their bioanalytical testing is phase-appropriate, sensitive, and scientifically justified.
If your strategy isn’t already built around high-quality analytics, now is the time to adapt. Read on to learn more about the changes in requirements for bioanalytical testing, and whether your current in vitro analytical methods might need a fresh look.
FDA's New Biosimilar Guidelines: A Strategic Shift Toward Bioanalytical Excellence
With fewer mandatory clinical trials, bioanalytical testing takes center stage. Your development strategy must now meet the FDA’s higher standard for:

1. Analytical Similarity
Deep molecular and functional characterization to prove equivalence.
2. Pharmacokinetics (PK)
Comparative PK profiles using validated ligand-binding or LC-MS assays that reflect how the product behaves in vivo.
3. Immunogenicity
Sensitive ADA methods, including screening, confirmatory, and neutralization tiers, aligned with risk-based strategies.
4. Functional Assays
Cell-based potency assays or binding kinetics (e.g., BLI) that tie your mechanism of action to therapeutic effect.
The end goal? A phase-appropriate, orthogonal bioanalytical package that replaces the need for comparative efficacy trials.

How Your Bioanalytical Tools Need to Evolve
The FDA is signaling: it’s not just about having data. It’s about having the right kind of data.
Here’s where common bioanalytical tools now stand within the new framework:
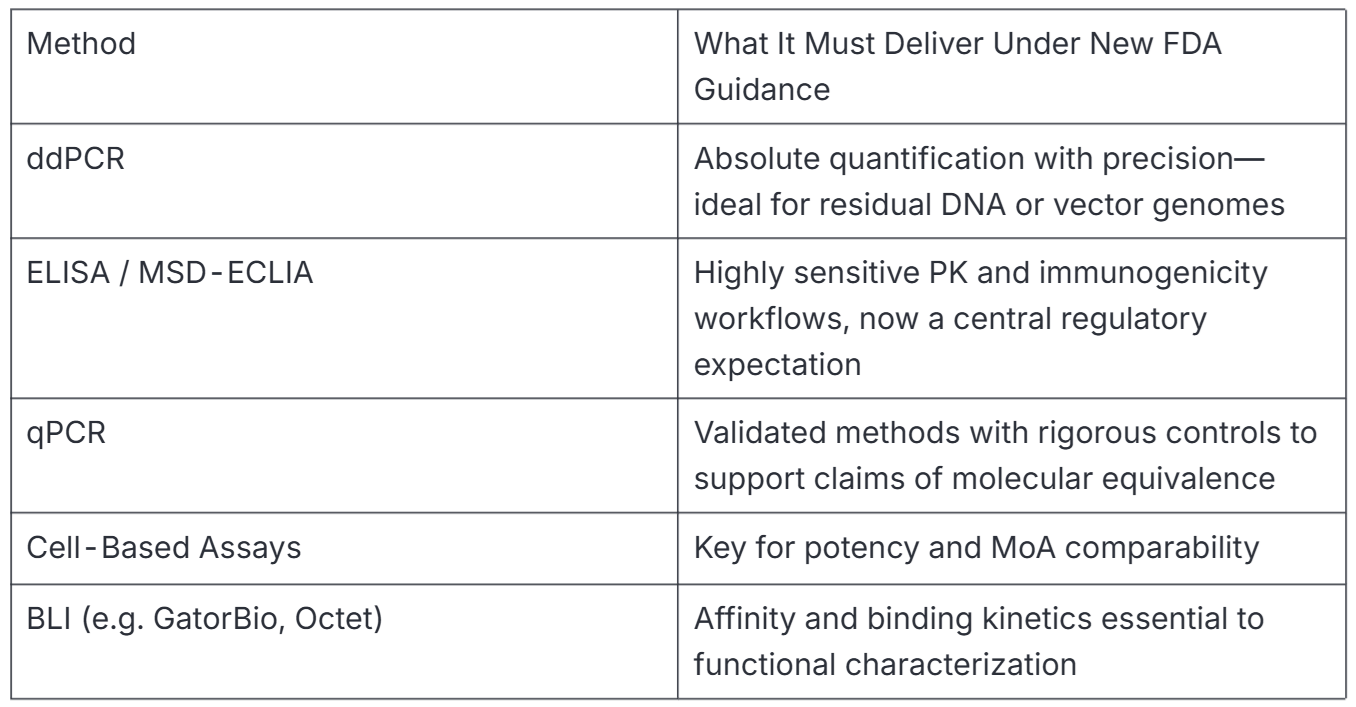
Role of ddPCR, ELISA, and qPCR
Working with a lab that specializes in bioanalytical testing for biosimilars can help you achieve this as well as strengthen your submission.
At Custom Biologics, we’ve spent over 20 years building a platform that reflects the exact types of GLP-/GMP-compliant bioanalytical services the FDA now expects for biosimilar approval without Phase III trials.
Our core areas map directly to the new regulatory focus:
- Cell-based potency assays & bioassays
- PK & ADA immunogenicity testing (ELISA, MSD-ECLIA)
- Binding kinetics via BLI (Octet Red96, GatorBio)
- ddPCR & qPCR genomics
- GLP-/GMP analytical validation and regulatory documentation support
If you’re restructuring your biosimilar or biologic development plan, this is the time to work with a lab partner who brings both assay expertise and regulatory alignment.

Want to Fast-Track Your Biosimilar Development Without Phase III Clinical Trials?
Download our new guide, Navigating FDA’s New Biosimilar Pathway: A Practical Guide to Replacing Clinical Efficacy Trials with Bioanalysis and get:
- A breakdown of assays that replace clinical efficacy studies
- A CMC, PK, and immunogenicity readiness checklist
- Tools to help you align your team, timeline, and testing strategy
Download Now
.png)



