As gene therapy continues to reshape the treatment landscape for rare and genetic diseases, adeno-associated viruses (AAVs) have emerged as one of the most widely used vectors for gene delivery. But behind every successful AAV-based therapeutic lies a robust preclinical Chemistry, Manufacturing, and Controls (CMC) strategy—one that begins with thorough characterization.
Among the most critical components of this strategy are AAV potency assays, which play a central role in evaluating the biological activity, efficacy, and consistency of your viral vector product. These assays are crucial for ensuring the quality and safety of gene therapies, as outlined in the FDA's guidance on potency tests for cellular and gene therapy products.

Understanding AAV Potency Assays
AAV potency assays are essential tools in the preclinical testing phase of gene therapy development. These assays provide critical information about the biological activity and efficacy of AAV vectors.
Why Potency Assays Matter in AAV Drug Development
Potency assays are not just regulatory requirements. They’re scientific necessities. AAV-based products are complex biologics, and minor changes in manufacturing conditions can significantly impact their efficacy. Regulatory authorities such as the FDA and Health Canada require well-characterized potency assays early in development to demonstrate that your product will perform consistently in patients.
In the preclinical CMC phase, potency assays help you:
- Compare different AAV constructs and production platforms
- Optimize dose selection
- Support lot release and stability studies
- Inform the design of pivotal toxicology and efficacy studies
Ultimately, potency is a critical quality attribute (CQA)—and it must be measured using scientifically sound, reproducible, and validated methods.
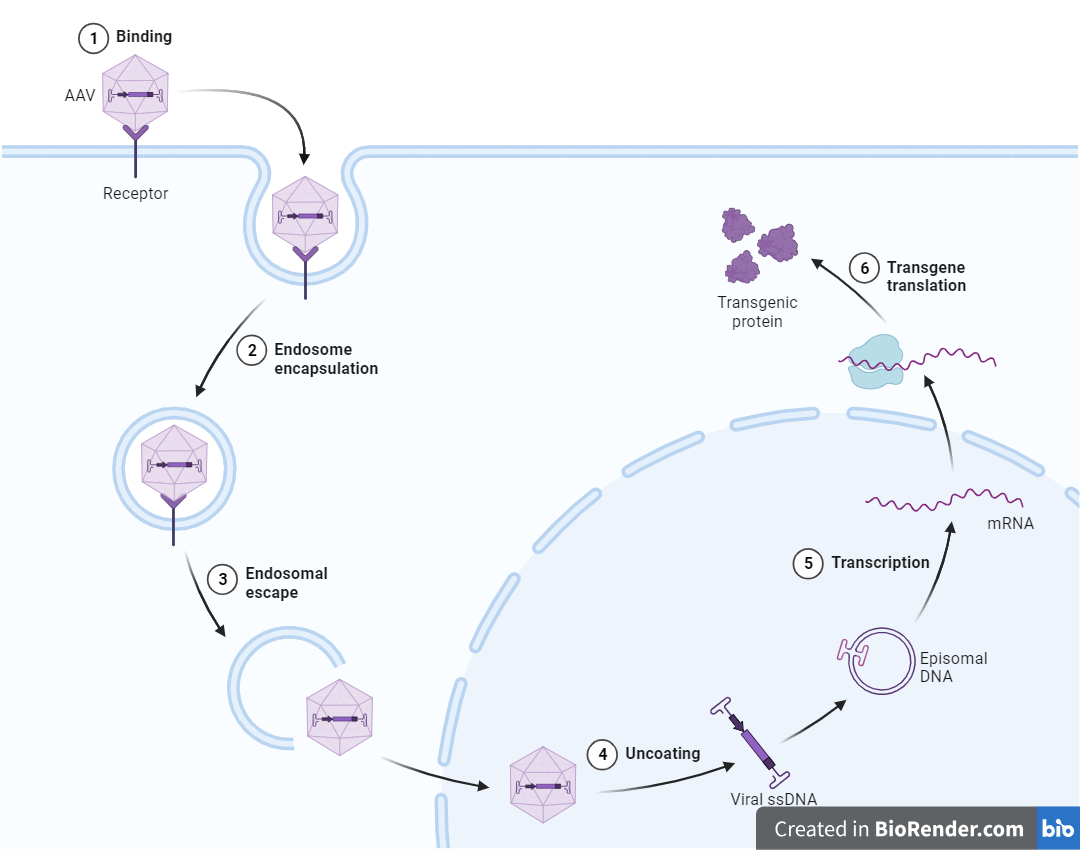
Challenges Characterizing AAVs
Characterizing AAVs for gene therapy development presents a number of technical and regulatory challenges. From early-stage research to preclinical CMC testing, pharmaceutical developers often encounter limitations in assay sensitivity, variability in biological activity, and evolving regulatory expectations.
Here are some of the most common challenges the industry faces:
- Potency Assay Development
Developing a reliable potency assay is challenging due to the variability in gene expression depending on the AAV serotype, transgene, and target cell type. The assay must reflect the intended biological activity and predict in vivo performance. - Genome Integrity and Packaging Efficiency
AAVs often contain a mix of full, empty, and partially filled capsids. Accurately measuring the proportion of full genomes is critical for dose consistency and safety, but methods like TEM (Transmission Electron Microscope) , AUC (Analytical Ultra Centrifuge), and ddPCR (Digital Droplet Polymerase Chain Reaction) are technically demanding and may yield different results. - Vector Heterogeneity
AAV products can show significant heterogeneity in capsid composition, post-translational modifications, and genome content, complicating both quality control and regulatory compliance. - Immunogenicity Risk Assessment
The immune system may recognize and respond to the AAV capsid or transgene product. Pre-existing neutralizing antibodies can limit efficacy, and there are no standardized methods to assess immunogenicity risk across different patient populations. - Low Abundance of Product
AAVs are often available in small quantities during early development, making it difficult to perform multiple analytical tests. Assays must be highly sensitive and optimized for small sample volumes. - Assay Standardization and Validation
Many analytical methods used to characterize AAVs, such as infectivity and potency assays, lack harmonization across labs. This makes method transfer and validation more difficult and can impact regulatory submissions. - Evolving Regulatory Expectations
As gene therapies continue to advance, regulatory expectations are rapidly evolving. Sponsors must stay current with guidance from agencies like FDA, EMA, and Health Canada to ensure their characterization strategy meets current standards.
Early development of a robust potency assay is critical to ensuring continuity between preclinical and clinical lots. By establishing a well-characterized assay early on, teams can track product consistency, support comparability, and streamline tech transfer. This foundation also helps accelerate scale-up and reduces the risk of regulatory delays due to inadequate or unqualified methods later in development.
Key Components of Potency Assays
Potency assays for AAV vectors typically consist of several key components, each designed to evaluate different aspects of vector performance.
- Transduction efficiency: This measures the ability of the AAV vector to enter target cells and deliver its genetic payload.
- Gene expression: Assays assess the level and duration of expression of the therapeutic gene carried by the AAV vector.
- Functional activity: These tests evaluate whether the expressed protein has the desired biological effect in relevant cell models.
Assay development should be phase appropriate, evolving in complexity and rigor as the program advances. In the early stages of the program, assays may be developed to demonstrate its feasibility and support process development of pilot batches. In the later stages of the program, qualification and/or validation of the methods to meet regulatory expectations are required. It is important to ensure that the validated methods are robust, reproducible, and fit for its intended purpose. Building this progression into your development plan supports both scientific integrity and regulatory compliance.
Researchers at Custom Biologics emphasize the importance of using a combination of these components to get a comprehensive picture of AAV vector potency.

Main Considerations When Developing an AAV Potency Assay
Developing a robust AAV potency assay requires careful consideration of multiple factors to ensure the assay is fit for purpose, especially in a regulatory context. Key elements include:
- Multiplicity of Infection (MOI): Choosing the right MOI to ensure sufficient transduction without over-saturating the system.
- Cell Line Selection: Using a biologically relevant, permissive, and consistent cell line that reflects the AAV’s mechanism of action.
- Quantitative Readout: Selecting a readout method (e.g., luminescence, fluorescence, qPCR) that directly reflects vector potency and therapeutic effect.
- Assay Sensitivity & Specificity: Ensuring the assay can detect small changes in potency while minimizing background or off-target signals.
- Reproducibility: Designing the assay to yield consistent results across analysts, instruments, and runs.
- Functional Degradation Tracking: Demonstrating that the assay can detect potency loss over time, supporting stability and comparability studies.
Common Platforms for AAV Potency Assays
At Custom Biologics, we support AAV analytics using a range of complementary techniques, including:
Cell-Based Potency Assays
These are often the gold standard for assessing transgene expression and function. They can be designed to measure a therapeutic protein’s biological effect in a relevant cell line, offering insight into functional potency under real-world conditions.
Flow Cytometry
Flow cytometry enables quantification of AAV transduction at the single-cell level. It’s particularly useful when evaluating serotype-specific transduction or cell-type selectivity.
ELISA & ECLIA
Used to measure secreted or intracellular levels of the therapeutic protein or related biomarkers. These immunoassays offer a high-throughput, quantitative readout of expression levels post-transduction.
qPCR and Droplet Digital PCR (ddPCR)
These methods quantify vector genome copies and support biodistribution, dose-response, and vector persistence studies. ddPCR is especially suited for gene therapy due to its sensitivity and reproducibility.
Gator BLI Technology
This technology quantifies AAV capsids by detecting real-time interaction between viral particles and specific anti-capsid antibodies immobilized on the surfaces biosensor probes.
Selecting the Right Assay Partner
Choosing the right CRO partner is essential to developing a potency assay that not only meets regulatory expectations but also accelerates your program’s progress.
Look for CROs that adhere to Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) standards. These guidelines ensure that all processes and procedures are well-documented and consistently followed.
A robust quality management system should be in place to handle all aspects of sample processing, data collection, and reporting. This system helps maintain data integrity and traceability throughout the testing process.
Regular audits and proficiency testing programs demonstrate a CRO's commitment to maintaining high-quality standards. These practices help ensure that the CRO's performance meets or exceeds industry benchmarks.
At Custom Biologics, we specialize in custom AAV potency assay development and bioanalytical services for gene therapy products. Our Canadian facility maintains a Quality Management System based on FDA GLP guidelines and is:
- Health Canada Drug Establishment (DEL) Licensed
- ISO 13485 compliant
- Recently Health Canada audited
With deep experience supporting preclinical and early-phase development programs, we work closely with clients to tailor assays that align with their specific therapeutic goals and regulatory pathways.



